THERDOSE PHARMA PRIVATE LIMITED (INDIA)
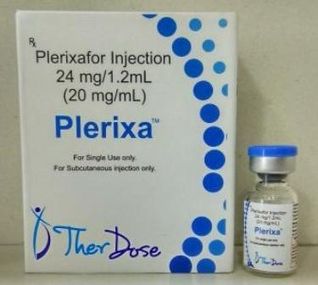
Plerixafor-plerixa
“Plerixafor” is the Drug / Molecule content in "Plerixa". Plerixa is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients into the bloodstream. The stem cells are then extracted from the blood and transplanted back to the patient
Indication
Plerixa is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with NHL and MM. The drug is approved for patients with lymphoma and multiple myeloma.
Note
Precaution
- Plerixa is a prescription drug and should be used under proper medical guidance and advice.
- To be sold by retail on the prescription of Oncologist only.
- This injection should not be used if it contains visible particulate matter. Do not use if cloudiness or precipitate is observed.
- Plerixa may cause fetal harm when administered to a pregnant woman.
Strength
20 mg
Packing
Vial
Storage
Store at room temperature (25 C). Do not freeze.